A polypeptide substrate moves into the active site of the enzyme. Initially chymotrypsin is synthesised as chymotrypsinogen inactive form.
Solved Use This Diagram Of The Chymotrypsin Active Site To Chegg Com
Four active forms of chymotrypsin C C1 C2A C2B and C3 were isolated from the autolyzed porcine pancreas glands.

Active forms of chymotrypsin is/are. Triose phosphate isomerase prenyl transferase and chymotrypsin are examples of enzymes that perfom isomerization condensation and hydrolysis respectively. There are two main forms of chymotrypsin such as chymotrypsin A and chymotrypsin B and they slightly differ in there structural and proteolytic characteristics. Their molecular weights were estimated by SDS-polyacrylamide gel electrophoresis to be 29 100 for C1 26 300 for C2A and C3 and 25 500 for C2B.
The active site of chymotrypsin is marked by serine 195. A chymotrypsin activator cleaves chymotrypsinogen to form active chymotrypsin which then hydrolyzes the non-fluorescent substrate to release a stable fluorophore ExEm 380460 nm. The optimum pH in which chymotrypsin acts is 78 80.
Consequently active sites of the enzyme are exposed which is called as alpha-chymotrypsin active form. Like most proteolytic enzymes chymotrypsin is activated from its inactive zymogen precursor chymotrypsinogen in presence of Trypsin. Lactin is a hormone that activates the mammary glands to secrete milk for the new born baby to feed.
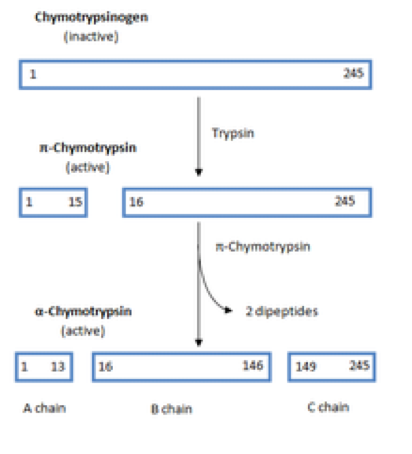
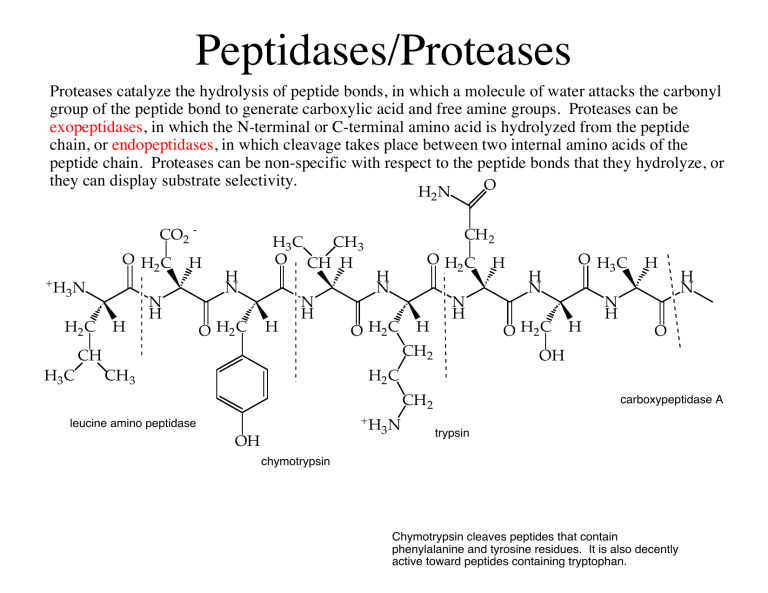
This active form is called π-chymotrypsin and is used to create α-chymotrypsin. It was also noted that chymotrypsin B in sharp contrast with chymotrypsin A splits acyl-tryptophan esters very slowly 20. The shape size and amino acid sequence of chymotrypsins active site allow that part of the enzyme to bind a portion of a polypeptide that has nonpolar side chains like those found in phenylalanine.
I will give an example to make you understand. Once released into the small intestine an enzyme found in the wall of the small intestine called enterokinase binds to trypsinogen and converts it into its active form trypsin. This creates two peptides within the π-chymotrypsin molecule held together by a disulfide bond.
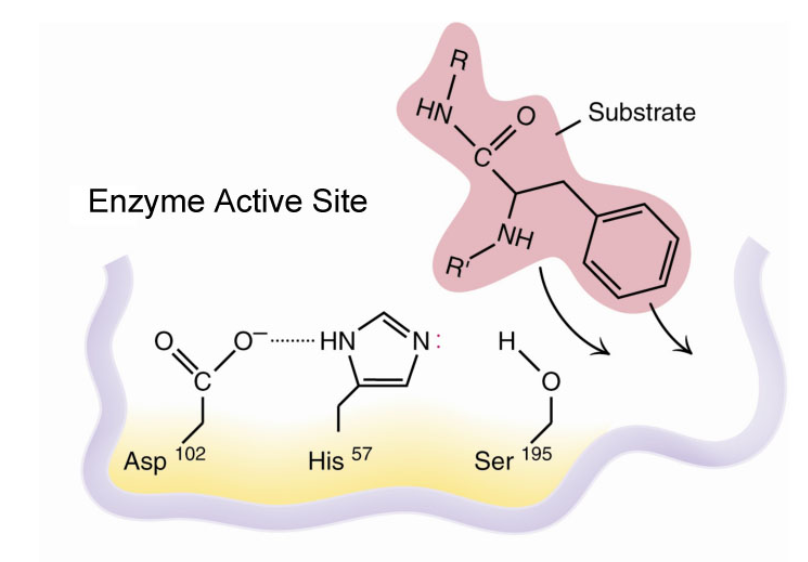
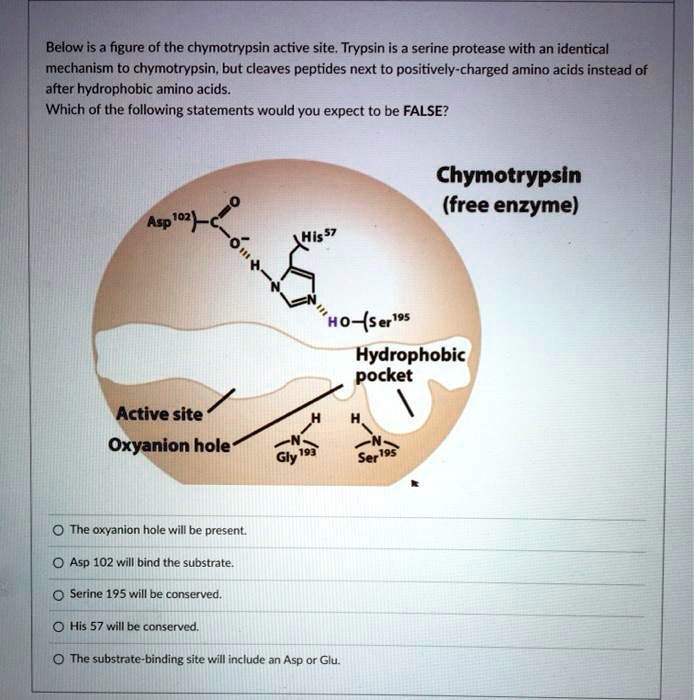
Chymotrypsin differs from trypsin in that trypsin cleaves peptides at arginine and lysine residues while. Serine is bonded to histidine 57 which is then bound to aspartate 102. Mechanism for chymotrypsin uses 3 amino acids at the active siteMechanism for chymotrypsin uses 3 amino acids at the active site Catalytic triad of serine histidine and aspartate H H O N N O O Serine Histidine Aspartic acid Chymotrypsin Oxford University Press 2013 9.
An enzyme lowers the activation energy of a reaction which means the transition state is not as energetically unfavorable as it. Trypsin then binds to chymotrypsinogen to convert it into the active chymotrypsin. The active site of chymotrypsin contains a catalytic triad and is composed of Histidine H57 Aspartic acid D102 and Serine.
Chymotrypsin EC 34211 chymotrypsins A and B alpha-chymar ophth avazyme chymar chymotest enzeon quimar quimotrase alpha-chymar alpha-chymotrypsin A alpha-chymotrypsin is a digestive enzyme component of pancreatic juice acting in the duodenum where it performs proteolysis the breakdown of proteins and polypeptides. Trypsinogen is proteolyzed to trypsin by the action of enterokinase a protease. Hydrolysis or dehydration reactions cleave a substrate into two products by adding or removing water.
Their molecular weights were estimated by SDS-polyacrylamide gel electrophoresis to be 29 100 for C1 26 300 for C2A and C3 and 25 500 for C2B. Chymotrypsin is the most abundant pancreatic proteases that represent up to 10-20 of the total protein synthesized by the exocrine pancreas. The kit includes a selective chymotrypsin inhibitor that can be used to measure specific chymotrypsin activity in samples containing non-specific proteases and.
What is the active form of chymotrypsin. Experiments are performed to determine the initial reaction velocity of an. Once released into the small intestine an enzyme found in the wall of the small intestine called enterokinase binds to trypsinogen and converts it into its active form trypsin.
In the pancreas vesicles store trypsin chymotrypsin and carboxypeptidase as trypsinogen chymotrypsinogen and procarboxypeptidase. Iio-Akama K Sasamoto H Miyazawa K Miura S Tobita T. To become active the zymogen must be converted into an active protein by proteolysis.
Chymotrypsin is activated through cleavage of the bond between arginine and isoleucine R15 and I16 by trypsin causing structural modifications and formation of the substrate binding site Sears 2010. It was agreed that chymotrypsin A π is the most active form and that further autolysis results in a gradual decrease of the catalytic activity 1214. Chymotrypsin is the active form and chymotrypsinogen is the inactive form.
Examples of this are the proteolytic digestive enzymes trypsin derived from trypsinogen chymotrypsin derived from chymotrypsinogen and carboxypeptidase A derived from procarboxypeptidase A. This hormone is produced in the form of prolactin. Serine lies in a small pocket on the surface of the enzyme.
Any hormone having pro as a prefix then it is understood that it is in its in. Trypsin cleaves the peptide bond in chymotrypsinogen between arginine-15 and isoleucine-16. Answer 1 of 6.
Evaluate which of the following statements isare true about enzymes and the transition from reactants to products. It is activated into its active form by another enzyme called trypsin. In the pancreas vesicles store trypsin and chymotrypsin as trypsinogen and chymotrypsinogen.
The structure of chymotrypsin selectively cleaves aromatic amino acids due to the hydrophobic pocket at the active site. Four active forms of chymotrypsin C C1 C2A C2B and C3 were isolated from the autolyzed porcine pancreas glands. It is cleaved by trypsin which undergoes conformational changes.
All three of these residues are hydrogen bonded at this pocket.

A Molecular Structure Of R Chymotrypsin B Schematic Representation Download Scientific Diagram

Chymotrypsin An Overview Sciencedirect Topics

Ctrc Active Protein Chymotrypsin Human Pancreas Active Protein Np 009203 2
Solved A Describe Based Upon What We Know About The Chegg Com
Learn About Chymotrypsinogen Chegg Com
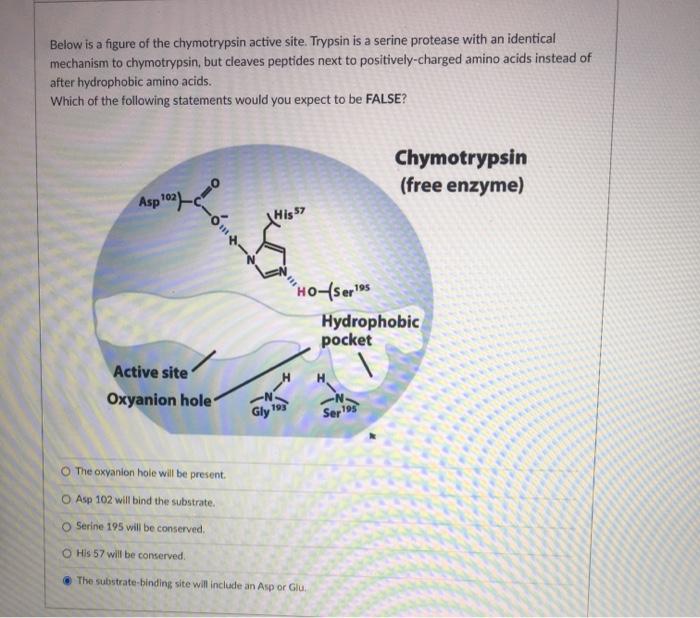
Solved Below Is A Figure Of The Chymotrypsin Active Site Trypsin Is A Serine Protease With An Identical Mechanism To Chymotrypsin But Cleaves Peptides Next To Positively Charged Amino Acids Instead Of After Hydrophobic

Chymotrypsin Knowledge Is Power Mark Bishop Active Site
Solved Below Is A Figure Of The Chymotrypsin Active Site Chegg Com

15 Catalytic Mechanism Of Chymotrypsin A Typical Serine Protease Download Scientific Diagram
The Chymotrypsin Trypsin Fold Consisting Of Two B Barrels And Eight Download Scientific Diagram
Peptidases And Chymotrypsin Mechanism Notes

Representative Scheme Of A Chymotrypsin Activation Residues That Download Scientific Diagram

The Pancreas Secretes The Zymogens Trypsinogen Chymotrypsinogen And Procarboxypeptidase Into The Lumen Gastrointestinal System Digestive System Biochemistry


Post a Comment
Post a Comment