This now-active form of pepsinogen generates pepsin from inactive pepsinogen by proteolysis. EL has a tropism for high.
Of note many key digestive enzymes such as α-amylase and lipase are present in the pancreas in their active forms.
Active form lipase. Two notable exceptions were the liver and the adipose tissue from fasted rats. Identification of the Active Form of Endothelial Lipase a Homodimer in a Head-to-Tail Conformation. Our data suggest the presence of two types of binding sites ie heparin-sensitive sites that bind primarily the catalytically active form of the lipase and are present at the endothelium in all blood vessels and heparin-insensitive sites that bind both active and inactive forms and are present only within the sinusoids.
Endothelial lipase EL is a member of a subfamily of lipases that act on triglycerides and phospholipids in plasma lipoproteins which also includes lipoprotein lipase and hepatic lipase. Presumably these enzymes would not cause pancreatic cellular damage if released into the pancreatic celltissue because there is no. In support ofthis ideaseveralgroupshavereported that thesizeof LPL as measured by density gradient ultracentrifugation is 110 kDa twice the size of LPL monomers 55 kDa.
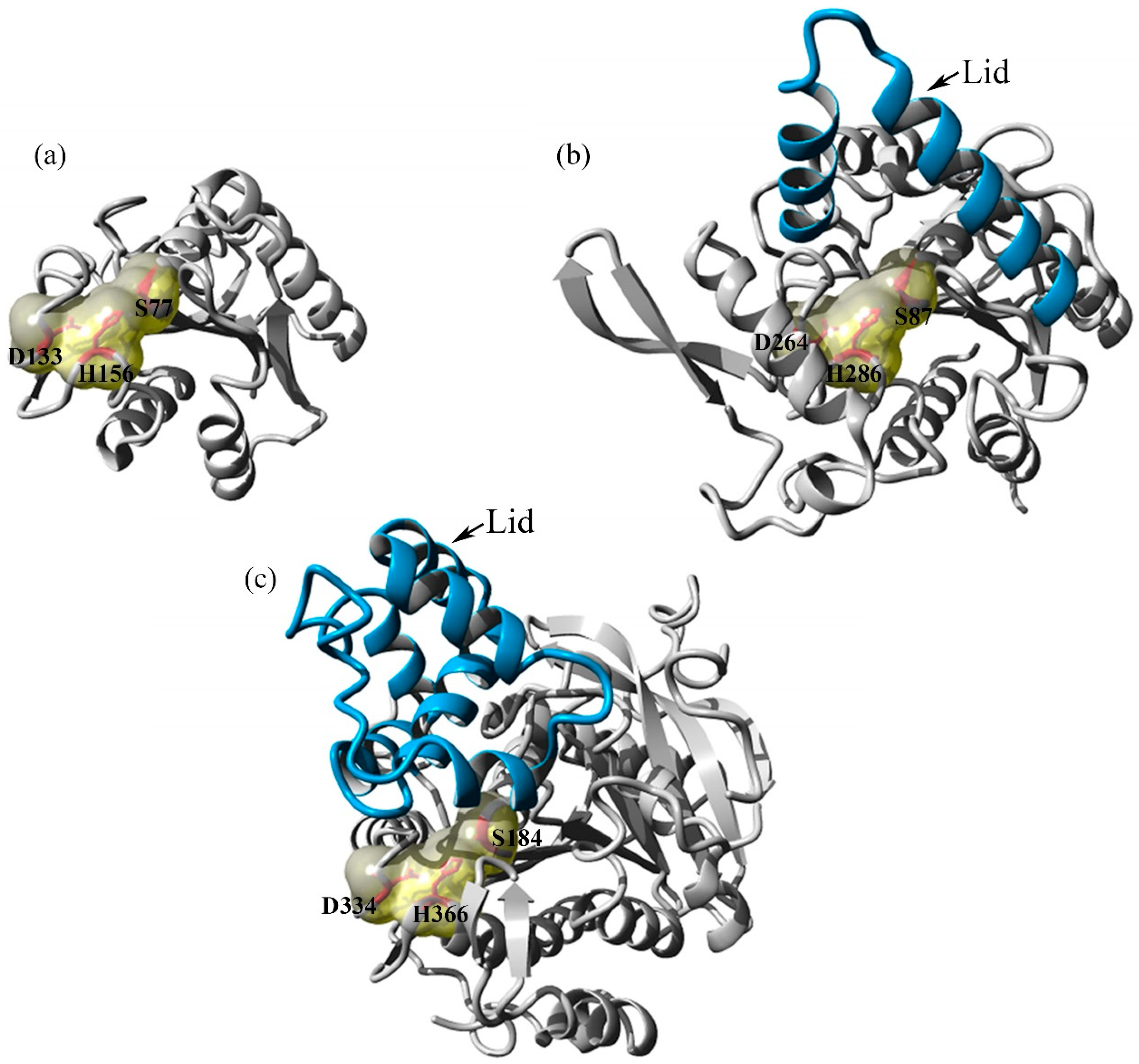
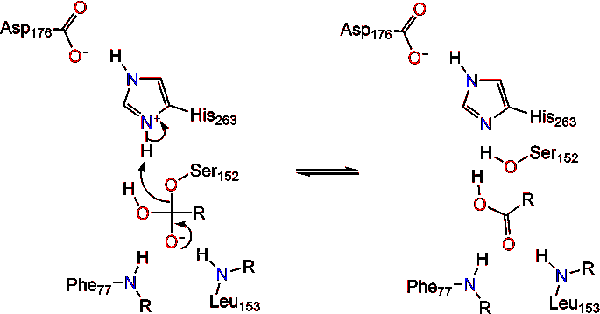
Immunoprecipitation experiments suggested oligomerization. In these studies the size and conformation of. A closed form in which the active center is hidden from the medium by a polypeptide chain called a lid and an open form in which the lid moves and exposes the active center of.
In general in presence of interface polarnon-polar the lid of lipase is dislocated and the enzyme shows its opened and active form which called interfacial activation behavior 2627 Moreover. Lipase bound to heparin-Sepharose could however be exposed to pH 45 at 10 degrees C with little loss of activity. Endothelial lipase EL is a member of a subfamily of lipases that act on triglycerides and phospholipids in plasma lipoproteins which also includes lipoprotein lipase and hepatic lipase.
Lipoprotein lipase LPL the enzyme that hydrolyzes triglycerides in plasma lipoproteins is assumed to be active only as a homodimer. Lipase is majorly synthesised by the pancreas and helps in the digestion of fats to free fatty acids and glycerol. The long form is expressed in steroidogenic tissues such as.
On secretion and exposure to stomach acid inactive pepsinogen undergoes a conformational change exposing its catalytically active site. Less of the LPL protein was in the active form in perfusates from hearts from fed compared with fasted rats. Binding of active lipoprotein lipase to heparin-Sepharose could be demonstrated at pH down to 65.
The results showed that the specific activity was high and similar in many of the tissues studied suggesting that the active form predominated. The enzyme has a long and a short form. Binding of active lipoprotein lipase to heparin-Sepharose could be demonstrated at pH down to 65.
Then we measured LPL mass and activity in a number of tissues. Shifted it forms a huge hydrophobic pocket exposing the ac-tive center to the medium resulting in the open and active form of the lipase with the hydrophilic phase of the lid inter-acting with the protein surface. The amount of lipase protein released remained relatively constant on changes in the nutritional state andor blockade of transcription but the distribution between the active and inactive forms changed.
In these studies the size and conformation of the active form of EL were determined. At pH below 6 binding could not be studied directly because the lipase was too unstable in. Hormone-sensitive lipase also previously known as cholesteryl ester hydrolase sometimes referred to as triacylglycerol lipase is an enzyme that in humans is encoded by the LIPE gene.
Extract and measure all forms of LPL from the tissues. Pepsin plays an important role in protein digestion during the gastric phase of digestion. Both conformational lipase forms.
A small amount of lipase in the blood is normal but the high level of lipase has clinical significance and it indicates an inflammation. In support of this idea several groups have reported that the size of LPL as measured by density gradient ultracentrifugation is 110 kDa twice the size of LPL monomers 55 kDa. Although hepatic lipase and lipoprotein lipase have been shown to function as homodimers the active form of EL is not known.
Lipoprotein lipase LPL the enzyme that hydrolyzes triglycerides in plasma lipoproteins is assumed to be active only as a homodimer. Endothelial lipase EL is a member of a subfamily of lipases that act on triglycerides and phospholipids in plasma lipoproteins. Lipase exists in two main forms open and closed.
Lipases are complex and special enzymes and have two different conformations. At pH below 6 binding could not be studied directly because the lipase was too unstable in solution. Although hepatic lipase and lipoprotein lipase have been shown to function as homodimers the active form of EL is not known.
EL has a tropism for high density lipoprotein and its level of phospholipase activity is similar to its level of triglyceride lipase activity. HSL is an intracellular neutral lipase that is capable of hydrolyzing a variety of esters. The active form trypsin then catalyzes the activation of the other inactive proenzymes.
Additional data obtained from aqueous solution activity measurements in the presence of detergent revealed that the fungal lipase retains an active conformation induced by high detergent concentration 30 mM for a long period of time a memory effect which is stabilized in the absence of a well-defined interface by few detergent molecules. In aqueous medium the lid or flap remains closed making it inactive while it remains open in the presence of natural substrates including oil converting it to an active form known as interfacial activation. Identification of the Active Form of Endothelial Lipase a Homodimer in a Head-to-Tail Conformation.

Three Dimensional Structure Of Human Pancreatic Lipase The Active Site Download Scientific Diagram

Pdf Physiological Regulation Of Lipoprotein Lipase Semantic Scholar

Pdf Lipases An Overview Methods And Protocols

Tertiary Structure For Horse Pancreatic Lipase Pl And Predicted Download Scientific Diagram

Scheme 2 A Enhanced Activities Of Lipases By Detergent Treatment Download Scientific Diagram

Catalysts Free Full Text Main Structural Targets For Engineering Lipase Substrate Specificity Html

Lipoprotein Lipase An Overview Sciencedirect Topics

Pancreatic Lipase Inhibitors The Road Voyaged And Successes Sciencedirect
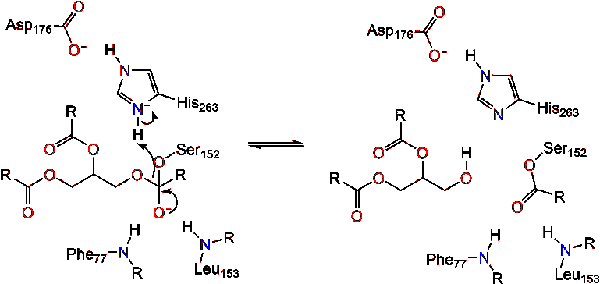
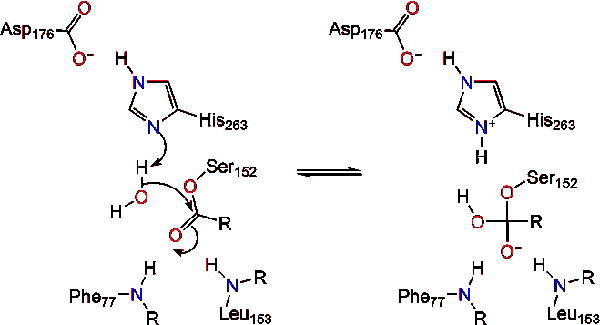

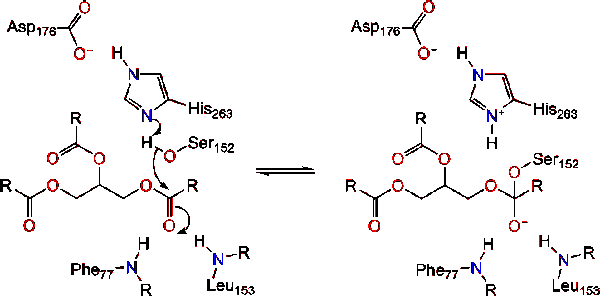

Post a Comment
Post a Comment